Abstract
Introduction
Ibrutinib, a Bruton's tyrosine kinase inhibitor, was approved in the U.S. for the treatment of relapsed or refractory mantle cell lymphoma (MCL) in November of 2013. However, real-world data on ibrutinib use for the treatment of MCL is limited. The purpose of this study was to examine ibrutinib use, dosages, and reasons for treatment discontinuation among MCL patients treated in a community oncology practice setting.
Methods
The study population consisted of adult (≥18 year old) MCL patients treated with ibrutinib between November 1, 2013 and October 31, 2016, who were not enrolled in a clinical trial and had at least 2 visits to a US Oncology Network (USON) clinic. Patients with other primary cancers were excluded. Patient data were sourced from the USON's electronic health records system, iKnowMed (iKM)™. The structured iKM database provided information on demographics and clinical and treatment characteristics. Manual chart review was used to confirm ibrutinib treatment patterns. Duration of ibrutinib therapy (DOT), overall survival (OS), and progression-free survival (PFS) from systemic treatment initiation were estimated using Kaplan-Meier methods. Events were defined as death in the OS analysis, and progression or death in the PFS analysis. Patients were censored if their treatment was ongoing for DOT. Censors for OS and PFS were patients lost to follow up or those who did not experience a failure event within the study period.
Results
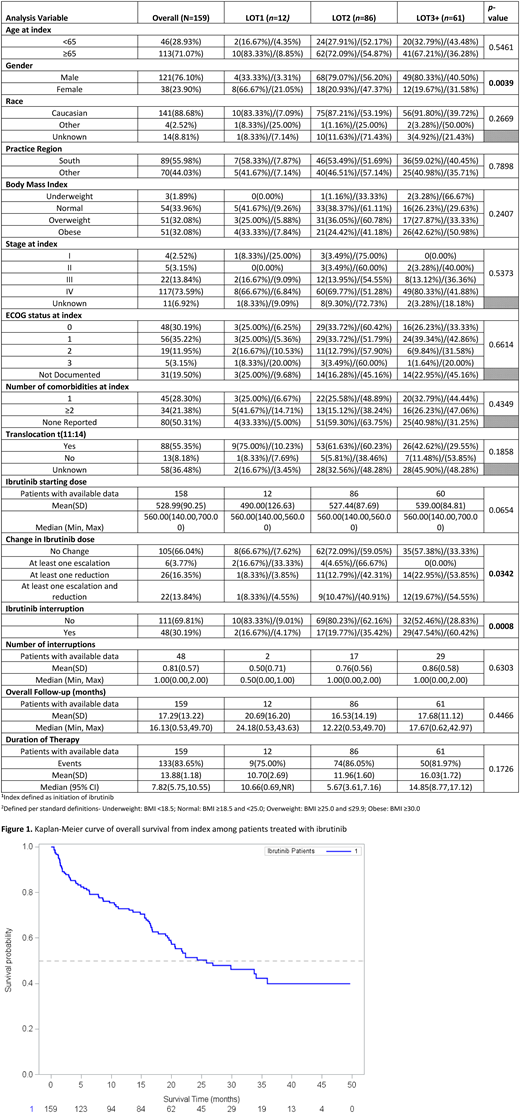
159 eligible MCL patients were identified through iKM. The majority of patients were Caucasian (n=141, 88.7%), male (n=121, 76.1%), and diagnosed with Stage IV disease (n=117, 73.6%). Median follow-up for the population was 16.1 months. Approximately 7.5% (n=12) of patients received ibrutinib as first-line therapy (1L), compared to 54.1% (n=86) in 2L and 38.4% (n=61) in 3L or beyond. Median ibrutinib dose at initiation was 560mg (range: 140-700). During ibrutinib treatment, 16.4% (n=26) of patients experienced a dose reduction. Dose holds occurred in 30.2% (n=48), 66.7% (n=32) due toxicities. The overall discontinuation rate was 83.6% The primary reason for discontinuation was disease progression (n=46, 34.6%) followed by toxicities (n=34, 25.6%). Median DOT was higher for patients initiating treatment in 3L+ (14.9: 95% CI 8.8-17.1) compared to other lines. Median PFS was 19.6 (95% CI: 16.5-24.3) for the overall population and median OS was 25.8 months (95% CI: 19.9-not reached).
Conclusions
Our real-world findings on survival are consistent with those from clinical trials on ibrutinib in relapsed/refractory MCL, although our observed discontinuation rate (~84%) was higher than that of the trial (~58%), which had a similar median follow-up time (16.1 months vs. 15.3 months, respectively). Our findings provide additional data on MCL treatment patterns and patient outcomes in clinical practice.
Sharman:Acerta: Consultancy, Research Funding; Pharmacyclics, an AbbVie Company: Consultancy, Research Funding. Kabadi:AstraZeneca: Employment. Clark:McKesson Specialty Health: Employment, Equity Ownership. Amirian:McKesson Specialty Health: Employment. Andorsky:Celgene: Research Funding; CTI BioPharma: Consultancy, Research Funding; AstraZeneca: Consultancy; Genentech: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal